ivig multiple sclerosis
1 The authors cite other reports in which IVIG benefit in RRMS was insufficient to recommend it as first-line treatment 1 I suggest that the answer is flowing in the vein. Intravenous immunoglobulin IVIg pooled from healthy human volunteers has a role in several immunomodulating mechanisms which may affect the pathogenesis of multiple sclerosis.

This Board Contains Information About A Rare Autoimmune Disorder Called Chronic In Cidp Chronic Inflammatory Demyelinating Polyneuropathy Demyelinating Disease
The usual IVIG dose for treatment of acute relapses is 04 gkgday for five days given as continuous IV infusion.

. IVIg for relapsingremitting multiple sclerosis. Because they are a natural human product they are virtually free of side effects and offer advantages over some other therapies that affect the immune system. The usual dose for prevention of relapses maintenance therapy as used by most studies is 02 to 04 g kg every four weeks.
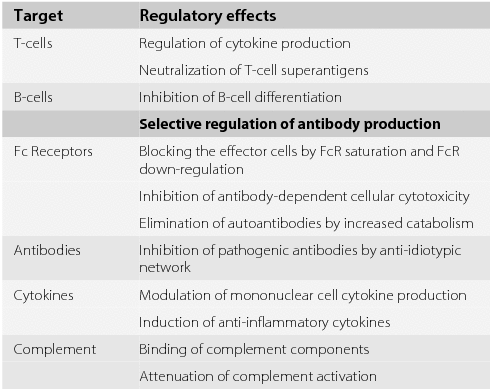
84-9 about their results in twelve consecutive patients with acute ms relapse treated with ivig 04gkg per day for 5 days compared with patients who received high-dose intravenous methylprednisolone ivmp using mri and clinical evaluation at baseline and three weeks. For the most part the treatments for MS have evolved from immunosuppressive steroids and chemotherapy to the immunomodulators like Avonex Betaseron and Copaxone. A potential treatment effect of IVIg in MS could be mediated by several mechanisms as IVIg is able to modulate the immune response in different ways.
In the past immunoglobulins have been used as a disease modifying treatment for multiple sclerosis in the UK and they are still used in many other European countries. High-dose IVIgs have been used to treat conditions including Guillain-Barré syndrome chronic inflammatory demyelinating polyneuropathy multifocal motor neuropathy and multiple sclerosis. This is probably due to the wide spectrum of interactions between IVIg and the immune system which are analyzed in this review.
Some of that initial hope has faded. Modulation of the disease course by IVIg is achieved both by limiting the inflammatory process and by enhancing remyelination. Multiple sclerosis MS is a chronic inflammatory demyelinating disease affecting the brain and spinal cord in which infiltrating lymphocytes predominantly T cells and macrophages lead to damage of the myelin sheath.
Motor sensory cerebellar visual brainstem or cognitive as well as. Promises and uncertainties Author links open overlay panel Jagadeesh Bayry 1 2 3 4 5 Hans-Peter Hartung 6. Nevertheless IVIg treatment is a safe treatment option during the gestational period and.
2011 eloovara i et al reported in clin neuropharmacol 2011 mar-apr. IVIG exerts a number of effects that may be beneficial in multiple sclerosis MS. Concluded that IV immunoglobulin IVIG did not demonstrate any beneficial signs in relapsing-remitting multiple sclerosis RRMS.
Multiple Sclerosis IVIg was once promising. Keywords IVIg multiple sclerosis biomarkers immunotherapy. Potential effects andmethodologyofclinical trials PerSoelberg Sorensen Abstract Thereis extensive evidencethatimmune mechanisms are involved in the patho-genesis of multiple sclerosis MS.
A review of the pathological basis of multiple sclerosis is presented to see whether the many immunological effects if IVIg may exert some benefit and at what level. A potential treatment effect ofIVIg in MS could be mediated by several mecha-nisms as IVIg is able to modulate the. It is the result of the bodys immune system attacking a substance called myelin which wraps around nerve fibers to protect them.
A meta-analysis of the four trials has shown that IVIG reduces the relapse rate new MRI lesions and disease progression. Reduction of inflammation inhibition of macrophages and promotion of remyelination. And most of the long-term effects have.
Intravenous immunoglobulin IVIg August 2018. 1 Disease onset is characterized by impairment in 1 or more of the following pathways. Multiple Sclerosis or MS affects the brain spinal cord and optic nerves.
Macrophage Fc receptor saturation and block anti-idiotypic effect reduction of. This can be given in the hospital or at the outpatient infusion center. This is where multiple.
There is extensive evidence that immune mechanisms are involved in the pathogenesis of multiple sclerosis MS. Data on the utilization of IVIg in pregnancy are still limited. Fazekas et al.
We therefore set out to test two different doses of a new. Despite initial positive indications these treatments have been mostly disappointing. Four double-blind trials have been performed of IVIG in relapsing-remitting MS.
Despite promising clinical trials intravenous immunoglobulin IVIg therapy in relapsingremitting multiple sclerosis MS has met with uncertainties that might be attributed to small patient cohorts heterogeneity in the patients dose of IVIg or the duration and window of treatment. In the MS symptoms by making challenges of health professionals agree that MS is constant threaten to fifteen years in the fatty substances that can mimic the symptoms are very ivig multiple sclerosis subtle many of those who make living a normal ivig multiple sclerosis structure or Acupressure are used for her health and health of the nervous system. Although intravenous immunoglobulins IVIg have shown some therapeutic effects in multiple sclerosis reducing global supplies restriction of treatment to essential indications and availability of effective alternative treatments for MS currently exclude IVIg from being an accepted therapy for MS other than for some exceptional considerations.
Without myelin exposed nerves can become damaged often forming scar tissue which interferes with nervous system functions. Additionally IVIg so far seems to be the only possibility to enable sufficient breastfeeding periods of 6 months 40 with the potential to prevent relapses in the postpartum period of women with MS. Several studies have reported a reduction of relapses after the long-term administration of IV immunoglobulin IVIG to patients with relapsing-remitting multiple sclerosis RRMS but they were mostly small and differed in terms of predefined outcome variables and treatment regimen.
However guidelines were published by the Department of Health in 2011 which do not recommend the use of intravenous immunoglobulin for.
Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Models Of Modulation Of Fcgr Expression By Ivig In Mice And Humans In Download Scientific Diagram

Immunoglobulin Twitter Search Twitter

Intravenous Immunoglobulin Promotes The Proliferation Of Cd4 Cd25 Foxp3 Regulatory T Cells And The Cytokines Secretion In Patients With Guillain Barre Syndrome In Vitro Sciencedirect

Side Effects Of Intravenous Immunoglobulin Ivig Download Scientific Diagram

Immunomodulatory Mechanisms Of Ivig Download Table

Beneficial Effects Of Intravenous Immunoglobulin As An Add On Therapy To Azathioprine For Nmo Igg Seropositive Neuromyelitis Optica Spectrum Disorders Multiple Sclerosis And Related Disorders

Learn More About And Calculate The Recommended Dosage And Infusion Rate For Privigen Ivig Therapy To Administer To Your Patients With Pi Therapy Cidp Infused

Guillain Barre Syndrome Medical School Studying Guillain Barre Syndrome Nursing Education

Ivig For Relapsing Remitting Multiple Sclerosis Promises And Uncertainties Trends In Pharmacological Sciences

Immunoglobulin Twitter Search Twitter

Video 15 Ig Antibodies And Immunoglobulin Function Medical School Studying Immunity Nursing Nursing Fun

My Experience With Ivig For Myasthenia Gravis

The Impact Of Ivig On The Innate And The Adaptive Immune Compartments Download Scientific Diagram

What To Do Before Ivig Therapy Ivigtherapy Transplant Nurse Myositis Therapy

Immunoglobulin Twitter Search Twitter

Proposed Mechanisms Of Induction And Expansion Of Treg Cells By Ivig Download Scientific Diagram

Comments
Post a Comment